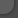|
Cytology of the CNS
3.1 Neurons
3.1.1 Morphology
3.1.2 Function
3.1.3 Pathology
3.2 Astrocytes
3.2.1 Morphology, Classification, and Immunoreactivity
3.2.2 Function
3.2.3 Pathology
3.2.3.1 Reactive Astrogliosis
3.2.3.2 Piloid Gliosis
3.2.3.3 Metabolic (Protoplasmic) Gliosis
3.2.3.4 Regressive Alterations
3.3 Oligodendrocytes
3.3.1 Morphology, Classification, and Immunoreactivity
3.3.2 Function
3.3.3 Pathology
3.4 Microglia and/or Mononuclear Phagocytes of the CNS
3.4.1 Morphology, Classification, and Immunoreactivity
3.4.2 Pathology
3.4.3 Function
3.4.3.1 Resting Microglia
3.4.3.2 Perivascular Microglia
3.4.3.3 Activated Microglia (Macrophages)
3.5 Additional Cell Types and Tissue Components
3.5.1 Leptomeningeal and Perivascular Cells
3.5.2 Parenchymal Vessels
3.5.3 Choroid Plexus and Ependyma
3.5.4 Pathology of the CSF and Cells within the CNS
Bibliography
Most standard textbooks of neurohistology and neurophysiology (e.g., Brodal 1998) contain both a general survey and detailed description of the morphology and function of the various cell types that make up the nervous system. Here we will only touch briefly on the essential points without attempting to provide a thorough overview. The term ”pathology” will be used here to denote the morphological changes exhibited by cells under pathological conditions and the reactions of various cell types to pathological changes within the nervous system.
3.1 Neurons
3.1.1 Morphology Neurons are simultaneously genetic, structural, functional, and trophic units. Characteristically large nuclei of neurons are spherical or oval, with clear nucleoplasm containing one or two distinct nucleoli (Fig. 3.1a). The state of neuronal activity is reflected in the structure of its nucleoli. Human brains contain about 1Ч1012 neurons and each cortical neuronreceives up to 30,000 presynaptic terminals (Tanner 1978). With increasing age the lipofuscin content of the neurons increases (Fig. 3.1b). If there is disruption of peripheral axons, the perikaryon will swell and the Nissl substance will disappear centrally, a process termed central chromatolysis (Fig. 3.1c). The cell body of the neuron, the perikaryon, is typically pyramidal in shape (Fig. 3.1a) and has long processes varying in number and length extending outward from it. There are usually several branched dendrites but only a single axon with terminal ramifications. Dendrites often possess spines or spikes that are sites of specialized, adjustable contact with other neurons. Intact dendrites are demonstrable by microtubule-associated protein (MAP) immunohistochemistry (Fig. 3.2a) and may be disrupted and destroyed by mechanical and ischemic influences (Fig. 3.2b). The axons are demonstrable by silver
Fig. 3.1a-c. Nerve cells. a Normal pyramidal nerve cells in the cerebral cortex; b lipofuscin-containing neurons of an aged man; c central chromatolysis. a, c Nissl stain; b H&E; magnification a-c Х1,000
staining (intact axons: Fig. 3.2c; disrupted axons: Fig. 3.2d). Axonal injury leads to a ball-like swelling of the disrupted axon which is seen in silver staining specimens (Fig. 3.2e) and after a-amyloid precursor protein (?-APP) immunohistochemistry (Fig. 3.2f). The cytoplasm of neurons is rich in organelles for oxidative metabolism and protein synthesis. The numerous mitochondria attest to the intense aerobic adenosine triphosphate (ATP) production using glucose as a substrate. The neuronal cytoplasm contains free ribosomes, lysosomes, Golgi complexes, and rough endoplasmic reticulum ? organized in so-called Nissl bodies ? for protein synthesis. The perikaryon also has a special cytoskeleton consisting of fibrillary proteins, especially actin filaments, microtubules, and intermediate filaments (neurofilaments) that manage cytoplasmic transport within the axons and dendrites. Such a system is necessary to transport proteins along the axons and to sustain the synapses. Stains used to demonstrate neurons are the Nissl method (Aniline dye) and silver staining techniques for demonstration of axons, dendrites, and/or spines (Golgi methods). Golgi methods usually stain only about 5% of neurons, which is to great advantage since the sections would be uniformly black, and uninterpretable if the silver precipitated through every neuron in a section. Axons are either myelinated or “unmyelinated.” The myelin sheath of myelinated axons (Fig. 3.3a) reduces the current loss between nodes of Ranvier, from the axons to ambient tissue fluid during impulse conduction, allowing the current to jump faster from node to node (see below). The velocity at which an impulse travels along the axon is proportional to the diameter of the axon and of the myelin sheath: axons with the thickest myelin sheaths conduct at about 120 m/s, while unmyelinated axons conduct at less than 1 m/s. The (pathological) demyelination follows ischemic or inflammatory insults and is marked by a loss of myelinated axons (Fig. 3.3b, c) and phagocytosis of myelin fragments (Fig. 3.3d). Peripheral nerves are surrounded by three layers of connective tissue that protect them from mechanical trauma: an external thick layer, the epineurium, an internal layer, the perineurium, and a layer of thin collagen fibers and fibroblasts, the endoneurium. A nerve impulse is conducted along the axon to its synaptic end, where chemically mediated transfer of signals between neurons takes place. Neurotransmitters (e.g., glutamate, norepinephrine or acetylcholine) reside in synaptic vesicles located near the presynaptic membrane of boutons and are released by exocytosis. From each packet released by the presynaptic bouton, only a few thousand molecules find a receptor before they disperse or are removed enzymatically or by re-uptake. Depolarization of the presynaptic membrane usually precedes transmitter release, which itself requires Ca2+ to enter the bouton. The number of quanta of transmitters released is directly proportional to the amount of Ca2+ entering the bouton. Neurons have three major cytoskeletal elements: neurofilaments, which are neuron-specific intermediate filaments that fill most of the axoplasm; microtubules, which are formed in the perikaryon and axon and serve the axonal transport system; and purified microtubuli, which consist mainly of ?- and ?- tubulin plus several polypeptides known collectively as microtubule-associated proteins (MAPs). The chief MAPs, MAP1 (350 kDa) and MAP2 (280 kDa) (see Fig. 3.2a), contribute to the assembly and stabilization of microtubules. MAP2 is a dendritic cytoskeletal molecule. Because no protein synthesis occurs in either axons or distal dendrites, the transport system allows anterograde and retrograde transport of proteins within axons and dendrites (Sotelo and Triller 1997). Although neurons are generally regarded as postmitotic cells, recent investigations have demonstrated “adult neurogenesis,” especially in the hippocampal dentate and olfactory bulb (for details, see p. 66).
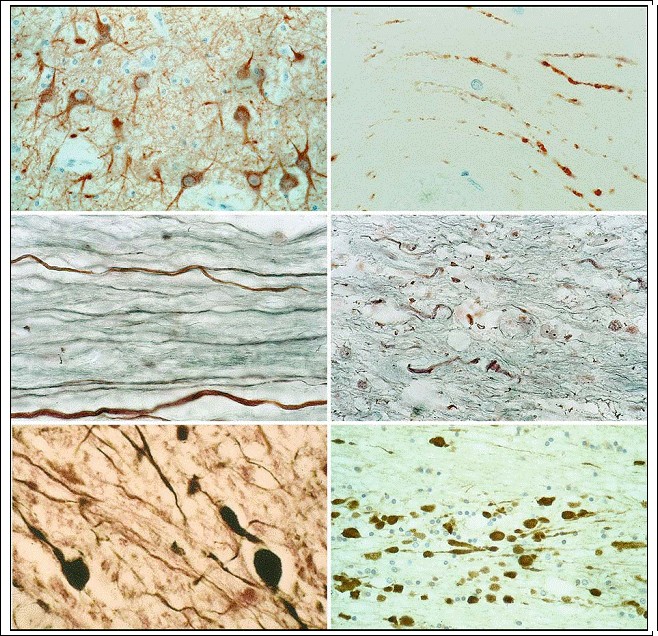
Fig. 3.2a?f. Neuronal processes. a Intact dendrites and b destroyed dendrites in the cerebral cortex (MAP2; magnification a X500, b Ч1,000); c intact axons and d disrupted axons (silver stain; magnification c, d Х1,000); e axon swelling/balls (silver stain; magnification Х1,000); f axon swelling/balls [?-amyloid precursor protein (?-APP) reactivity; magnification Х500]
3.1.2
Function Neurons are the principal transducing cells of both the central and peripheral nervous systems. Though they exhibit great structural variation, they all serve the same purpose: to receive, process, and transmit information via bioelectric signals (Kreutzberg et al. 1997). Neurons are characterized by their excitability and ability to conduct impulses, i.e., if sufficiently stimulated they release a brief electrical discharge, termed an action potential, which is conducted along the axon. The action potential is a major constituent
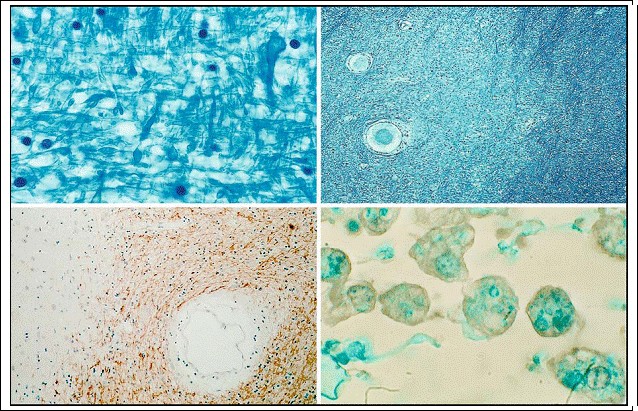
Fig. 3.3a?d. Myelin. a Normal myelin-staining pattern and b focal demyelinated structures in white matter of the cerebrum (Luxol fast blue; magnification a х1,000; b х100); c local demyelination in white matter as seen by application of antibodies against myelin basic protein (= MBP; magnification х200); d the demyelination is characterized by phagocytosing cells (activated microglia) which contain myelin fragments (Luxol fast blue; magnification х1,000)
of communication among nerve cells and between nerve cells and the body; it is an indispensable link between the central nervous system (CNS) and the world around us. An action potential is created by the movement of ions through the cell membrane, which requires an electrical potential between the interior and exterior of the cell, the membrane potential. The electrical current is transformed at the synaptic level into a chemical signal: the released transmitters bind briefly to receptors on the postsynaptic membrane. The action potential is based on the presence of voltage-gated Na+ channels that open when the membrane is depolarized. Depolarization can result from electrical stimulation or opening of transmitter- gated Na+ channels. The latter induces a flow of Na+ into the cell driven by the concentration gradient and membrane potential. The inflow of Na+ ions ceases once the membrane depolarizes to +55 mV. The action potential is the result of an invariable all-or-nothing phenomenon. Messages can only be varied by a variation in the rate of action potentials, which in turn depends on the degree of depolarization. Some neurons are capable of a maximum frequency of action potentials exceeding 100 per second (100 Hz). The velocity at which an impulse is conducted depends on the axon‘s diameter and myelin thickness and in meters per second is roughly 6 times the axonal diameter in microns. An action potential is generated when positive charges penetrate to the proximal part of the axon, which at that point becomes positive relative to more distal parts along its length. A corresponding current of positive charges moves in the opposite direction outside the axon, thus establishing an electrical circuit. The action potential in myelinated axons spreads passively (electronically) to the first node of Ranvier and is regenerated at each further node, where the axon membrane lacks a myelin sheath. The action potential moves by “jumping” from one node of Ranvier to the next. Several authors have studied the role of neurons in the immune response process (Sedgwick and Hickey 1997). It is thought that healthy neurons probably do not respond even to cytokines, such as interferon-? (IFN-?), which are associated with the major histocompatibility complex (MHC). On the other hand, following infection, MHC expression has been found in neurons in vivo. In the healthy CNS, MHC class I and II molecules are virtually absent. Under pathological conditions, however, MHC molecules are known to be upregu- lated by various cells within the CNS (see below). Under normal circumstances neurons are able to prevent and/or limit inflammatory responses (for review see Neumann 2000, 2001). Under pathological conditions such as mechanical brain injury, genes are turned on, inducing a proinflammatory milieu with upregulation of MHC molecules, local production of proinflammatory cytokines, and recruitment of inflammatory cells (Streit et al. 1989; Olsson et al. 1992).
3.1.3
Pathology
Two apparently related types of cell death are to be distinguished: necrosis and apoptosis (pp. 62f). In addition to these degenerative changes of the cells themselves, pathological processes can be initiated by non-specific damage to axons and dendrites, as well as by regenerative processes. Since these negative and positive types of reactions affect all nervous tissue, not just neurons, they will be discussed in Chap. 4, “Cell and Tissue Reactions” (pp. 42ff). Ischemic cell necrosis is a specific pathological reaction of the neuron and is described in detail in Chap. 13. For details on the morphology and pathogenesis
of the other specific pathological changes of neurons, the reader is referred to the relevant neuropathology textbooks (especially Haymaker and Adams 1982; Graham and Lantos 2002; Peiffer et al. 2002). Let it be noted here, however, that in most cases diagnosis is based not on changes of the neurons alone, but on those changes in relation to all other trauma-induced tissue changes, i.e., in microglial cells, neuroglia, blood vessels, etc. Neurons undergo certain physiological changes, notably the accumulation of lipofuscin in neurons (Fig. 3.1b) of the dentate nucleus of the cerebellum and inferior olive, and in motor neurons in the anterior horn of the spinal cord. Such changes are especially common in brains of the elderly. Neurons also react to axonal injury in the form of retrograde degeneration, i.e., a central chromatolysis or ballooned neurons (Fig. 3.1c). A chronic cell change is characterized by a shrunken, intense, dark staining neuron. Further degeneration processes will be described below. But the following phenomena should be mentioned at the beginning:
- Loss of dendrites (and their MAP reactivity ? see Li et al. 1995, 1997) by ischemic or mechanical loading (Fig. 3.2b).
- Destruction of axonal fibers as demonstrated by silver technique (Fig. 3.2d).
- Axonal swelling as a result of an axonal lesion ? as demonstrated by the silver technique (Fig. 3.2e) and reactivity to ?-amyloid precursor protein (?- APP) (Fig. 3.2f).
- Destruction and loss of myelin is demonstrable both by loss of myelin staining and an increase in scavenger cell (macrophages), the latter indicating active demyelination (Fig. 3.3d).
Intracytoplasmic Storage. Neurons can store other substances besides lipofuscin. A new classification of the storage diseases was recently introduced based on the intracytoplasmic increase in gangliosides or ceroid lipofuscin in neurons (for details: see textbooks on clinical neuropathology).
Basophilic Inclusions (Lafora Bodies ? In Familial Myoclonic Epilepsy). Basophilic inclusions are globular bodies located within the cytoplasm. They vary in size from 1 ?m to 20 ?m, pushing the nucleus and Nissl bodies to the periphery of the perikaryon. The body is homogeneous in places, structured in others, and liable to splintering. If present in large numbers, they are indicative of a familial disease (myoclonic seizures, cerebellar ataxia). In rare cases, solitary Lafora bodies are present in normal postmortem material.
Inclusion Bodies in Viral Diseases. Inclusion bodies associated with viral diseases are usually intranuclear. In rabies, however, cytoplasmic inclusions are seen, termed Negri bodies, virus factories located in the cytoplasm of nerve cells of rabies victims (pyramidal cells of the hippocampus, Purkinje cells, and large motor cells of the brain stem and spinal cord). These are roughly spherical or oval bodies that range from 0.5 to 4.0 ?m in diameter. Intranuclear inclusion bodies are the norm in viral illness, and are encountered in neurons and glial cells (oligodendrocytes, ependymal cells) of patients with cytomegalovirus infection, progressive multifocal leukoencephalopathy, subacute sclerosing panencephalitis, or herpes simplex virus encephalitis. They possess an amorphous spherical structure that is homogeneous or granular and surrounded by a small clear zone of nucleoplasm.
Neuronal Vacuolation (Bovine Spongiform Encephalopathy = BSE). The large neuronal vacuoles have thin walls and appear to coalesce. They almost completely displace the perikaryon.
3.2
Astrocytes
Astrocytes are the most common cellular elements of the brain, outnumbering neurons by ten to one and taking up about one-third of the volume of the cerebral cortex (Pope 1978). Astrocytes, like oligodendrocytes, are “neuroglial cells” or “macroglial cells.” The two cell types derive from different precursors. These precursors, however, are the progeny of a common ancestor, the glioblast. Before birth the glioblast resides in the ventricular layer, after birth in the subependymal layer (Levison and Goldman 1993).
3.2.1
Morphology, Classification, and Immunoreactivity
A specific cell structure, immunoreactivity, and function characterize “astrocytes” as a distinct cell type. Morphologically they are distinguished by long, sometimes branched processes found in gray and white matter. Astrocytes are interconnected with each other via gap junctions that create a syncytium allowing ionic and metabolic coupling (Norenberg 1997). Endowed with receptors to most neurotransmitters and neuropeptides (Murphy and Pearce 1987), astrocytes possess messenger systems that maintain essential communication between themselves and neurons. Among the chief biological markers of astrocytes, besides glial fibrillary acidic protein (GFAP), are glutamine synthetase, S-100 protein, and pyruvate carboxylase. All those cell types possess 10-?m intermediate filaments. Those in astrocytes express nestin, vimentin, and GFAP (49-kDa protein) (Eng et al. 1971; Galou et al. 1996; Colucci-Guyon et al. 1999). Thus vimentin and GFAP, for example, coexist in immature and in reactive astrocytes (Eng and Lee 1995). As stated above, astrocytes also express glutamine synthetase and S-100 protein. Some astrocytes are GFAP negative (GFAP-negative astrocytes), chiefly in the fetal nervous system and in gray matter of the adult brain (Kitamura et al.
1987). In normal gray matter, these protoplasmic astrocytes contain only sparse GFAP and do not stain for GFAP in routine paraffin material. Astrocytes are of three basic types: fibrous and protoplasmic astrocytes, and radial astrocytes. The former two types are demonstrable in adult CNS; the first type is most frequently found in the white matter (fibrous type), the second in the gray matter (protoplasmic type). Radial astrocytes are found in the walls of cerebral vessels and the neural tube mainly
during embryonic development. The three types of astrocyte can be classified, and exhibit the following features (Privat et al. 1995): 1. Fibrous astrocytes (white matter astrocytes ? Fig. 3.4a,b). Markedly fewer in number than radial astrocytes, they are stellate in structure with long, thin, poorly ramified processes that are smoothly surfaced. The nucleus is spherical or oval shaped. Fibrous astrocytes react with GFAP antibody, but the cell body stains incompletely.
2. Protoplasmic astrocytes (gray matter astrocytes ? Fig. 3.4c). Protoplasmic astrocytes have ramified processes of variable caliber. Most astrocytes residing in normal gray matter are GFAP negative, and this is the basis for determining reactive gliosis. GFAP-positive astrocytes in the gray matter are small and possess many short processes radiating from the cell body, which is usually poorly marked by GFAP immunoreactivity.
3. Radial astrocytes (white matter astrocytes). These cells, disposed in a plane perpendicular to the axis of the ventricles, are of particular importance during CNS embryology (Rakic 1995; Parnavelas and Nadarajahn 2001). The nucleus and perikaryon of radial astrocytes are located in close proximity to the pia mater, especially in the cerebellum and spinal cord. The processes lack
ramification and at least one process touches the pia mater, the others coursing through the gray matter. GFAP-positive fibrous processes are distributed in the white matter of the lateral and ventral fasciculi of the spinal cord.
3.2.2
Function
Astrocytes are essential not only for keeping the highly differentiated neurons in their proper place within the brain, but also for maintaining their physiological environment (Lugaro 1907). It is known (Newman 1995) that a highly regulated intercellular environment is required for neuronal function and that astrocytes regulate the crucial environmental homeostasis of electrolytes, water, and pH and which eliminate amino acids and proteins from the extracellular space. Although primarily the role of endothelial tight junctions, astrocytes also uphold the blood?brain barrier (BBB) from extracerebral influences by controlling and regulating the intercellular transport of molecules from the vessel to the neuron. Astrocytes perform the following functions:
1. Developmental function (neurotrophic action). Astrocytes are indispensable for neuronal survival, migration, and neurite outgrowth. GFAP-negative astrocytes constitute a better substrate for
such functions than GFAP-positive astrocytes. This phenomenon may explain why astrocytes do
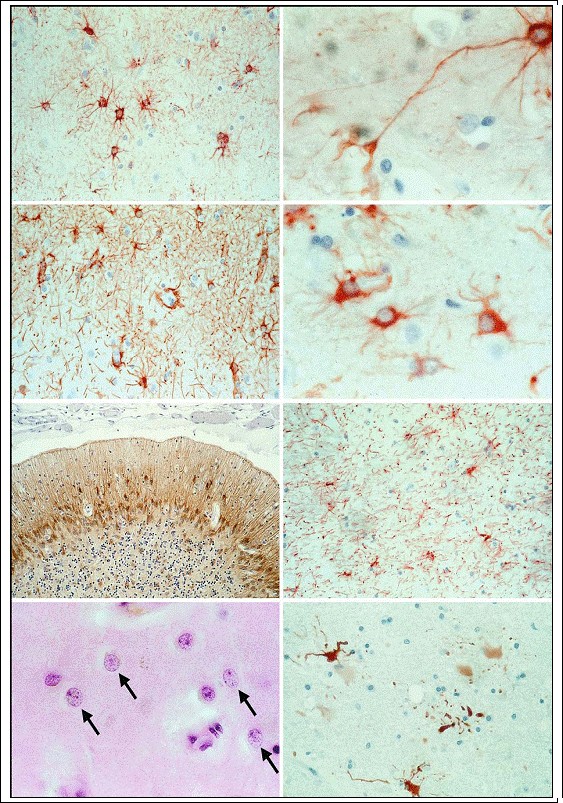
Fig. 3.4a?h. Astrocytes. a, b White matter astrocytes, i.e., fibrous astrocytes (GFAP; magnification a Ч500; b Х1,000); c graymatter astrocytes, i.e., protoplasmic astrocytes (GFAP; magnification Х300); d activated, plump (hypertrophic) astrocytes (GFAP; magnification Х1,000); e isomorphic gliosis (GFAP; magnification Х300); f anisomorphic gliosis (GFAP; magnification Х300); g Alzheimer type II astrocytes marked by arrows (H&E; magnification Х1,000); h clasmatodendrosis of GFAP-positive astrocytes (GFAP; magnification Х1,000)
not express GFAP until relatively late in CNS development after the early phase of neurogenesis has been completed (Menet et al. 2000). Astrocytes also promote myelin synthesis and remyelination (Franklin et al. 1993). 2. Electrolyte and water homeostasis and osmoregulation. Depolarization of neurons is achieved by a cellular efflux of K+ from active neurons with a consequent increase in extracellular K+. Astrocytes possess mechanisms for active and passive accumulation of K+ in the intercellular space and the transfer of K+ by spatial buffer currents to the capillaries and/or cerebrospinal fluid (CSF) space (Newman 1995). As stated above, astrocytes regulate not only K+ homeostasis, but also the homeostasis of Cl? and bicarbonate (Walz 1995), Na+ (Ballanyi 1995), Ca2+ (Finkbeiner 1995), and the pH (Deitmer 1995). Astrocytic swelling, known as cytotoxic edema (see below, p. 47), occurs almost immediately following the incidence of CNS injury and has been described in experimental allergic encephalitis (Eng et al. 1989). Axonal swelling probably arises from a trauma or disease-induced increase in levels of potassium, glutamate, fatty acids, arachidonic acid, lactic acid, and free radicals.
3. Astrocyte-neuron lactate shuttle. According to a recent published hypothesis (Hertz 2004), neuronal activity-induced uptake of glucose takes place predominantly in astrocytes, which metabolize glucose anaerobically while lacate produced from anaerobic glycolysis in astrocytes is then released
from astrocytes and provides the primary metabolic fuel for neurons (Chih and Roberts 2003, Hertz and Dienel 2005).
4. Control of vascular tone. Zonta et al. (2003) suggest that neuron-to-astrocyte signaling in the cerebral cortex is central to the dynamic control of vascular tone, and that astrocytes play a crucial role in this process. This conclusion is based on the fact that following electrode stimulation of neuronal afferents Ca2+ levels increase in the somata and endfeet of astrocytes linked to arterioles. Thus there is a bridge between the response of astrocytes to neural activity and the observed dilation of arterioles (cf. Reilly 2003).5. Transmitter inactivation mechanism. Henn and Hamberger (1971) demonstrated the uptake of ?-aminobutyric acid (GABA), norepinephrine, dopamine, and serotonin by a cell fraction rich in glial cells, suggesting that glia can eliminate, i.e., take up and metabolize, transmitters that overflow from the synaptic cleft. It is now known that protoplasmic astrocytes in the gray matter perform this function for the aforementioned amino acids and for the excitatory amino acid glutamate, inhibitory adenosine and adenosine triphosphate, histamine and N-acetylaspartylglutamate (Martin 1995).
6. Plasma protein uptake. Astrocytes immunostain for albumin (Klatzo et al. 1980), a phenomenon which has prompted some authors to propose that astroglial ingestion of plasma protein might aid in the resolution of brain edema (Oehmichen et al. 1979a; Tomimoto et al. 1996; Del Bigio et al.2000).
7. Reactivity in CNS injuries. Following mechanical violence to the CNS, astrocytes undergo specific proliferative, morphological, and biochemical changes termed astrogliosis or reactive gliosis (see below, pp.23f).
8. Immunological activity (for review see Dietrich et al. 2003). Astrocytes are stimulated by the
cytokines interleukin-1 (IL-1), IFN-? and tumor necrosis factor-? (TNF-?), as well as by multiple other growth factors (Norenberg 1997). Enlarged (reactive) astrocytes harbor an enhanced number of cytoplasmic organelles plus increased levels of GFAP, Ia antigen, IL-1, ?-1-anti-chymotrypsin, and acute phase reactive protein (Eddleston and Mucke 1993). Under pathological conditions (infiltration by activated T-cells, blood?brain barrier disruption), the CNS shows an increased expression of the class I/II MHC, the adhesion molecule ICAM-1, the TNF-? receptor and complement component C3 (see Morgan 1999) plus production of TNF-? and IL-6 (Benveniste 1997). Astrocytes release various neuroactive compounds when stimulated by neurotransmitters, compounds such as taurine in response to ?- adrenergic stimulation or GABA after glutamate receptor stimulation. A survey of the immune factors synthesized and released by astrocytes ? and their effects ? was published by Norenberg (1997).
9. Regenerative CNS processes. Gliosis clearly has an inhibitory effect on regeneration of the adult mammalian CNS (Fitch et al. 1999). However, there is also evidence that astrocytes play an active role in both embryonic and adult neurogenesis (Reilly 2002; Song et al. 2002; Svendsen 2002; for details see p. 66).
10. Neuron-like function. Recent evidence suggests that glial cells play more sophisticated, neuronlike roles; they integrate neuronal input, modulate synaptic activity, and process signals related to learning and memory (Kurosinski and Gцtz 2002).
3.2.3
Pathology
Astrocytes react differently to different neuropathological conditions, including trauma, infection, seizure, infarct, metabolic processes, and tumor infiltration. GFAP upregulation and fibrillogenesis are the principal factors underlying the formation of the glial scar.
3.2.3.1
Reactive Astrogliosis
Reactive astrocytes typically undergo enlargement of the (homogeneously stained) cell body and filaments. Cells and processes both increase strikingly in number. The increase of the processes may culminate in sclerosing gliosis. Astrogliosis is primarily characterized by swelling of the cell body and upregulation of glial filament expression (GFAP, vimentin). If cytoplasm is abundant and the cell rounded, such cells are variously termed “plump” astrocytes, “fattened” astrocytes or “gemistocytic” astrocytes (Fig. 3.4d). This cell type exhibits a homogeneous cytoplasm and a slightly enlarged nucleus with angular projections from
which the processes arise. The nucleus is lateralized to one side of the cell body and may be irregularly rounded. Astrogliosis is induced by transmembrane signals (e.g., growth factors and neuropeptides) via extracellular signal-regulated kinase and mitogenactivated protein kinase (Mandell 2001). In certain respects, reactive astrocytes resemble neurons undergoing central chromatolysis, which is a basic reaction to axonal transection (Bignami and Dahl 1995 ? see below, p. 66). Moreover, at sites of brain tissue destruction, astrocytes form a scar in which they begin to shrink after some time and finally disappear, leaving behind a dense meshwork of glial fibers (Weil 1933). Clean excision of the brain parenchyma will leave a fluid-filled space the surrounding wall of which contains astrocytes that have undergone only a slight reactive change. Laceration as well as hypoxic changes (see below) of the brain parenchyma produce a powerful glial response and also proliferation of connective tissue. Gliosis may be isomorphic or anisomorphic. Isomorphic gliosis (see also piloid gliosis ? Fig. 3.4e) typically occurs in cases of selective damage to neurons and their processes in which the glial fibers maintain their normal orientation. Anisomorphic gliosis (Fig. 3.4f) is seen in cases of severe brain damage with major disruption of the brain and glialarchitectonics with consequent disruption of the blood?brain barrier. Under pathological conditions glial cells form accessory glial limiting membranes. Reactive astrocytes, for example, may surround the necrotic tissue created by an infarct with a thick mesh of fibrils, thus demarcating it from viable brain tissue and helpingto thwart the spread of edema. This glial scar (Figs. 9.10?9.12) will eventually be covered by a basal lamina and form a new surface of the brain. The cyst produced as macrophages absorb the necrotic tissue becomes part of the subarachnoid space. Astrocytes contribute to the synthesis of chondroitin sulfateproteoglycan (Gallo and Bertolotto 1990) and of the basal membrane proteins laminin and fibronectin (Price and Hynes 1985; Liesi and Risteli 1989). Reactive astrocytes are characterized by a rapid synthesis of GFAP and ? to a lesser degree ? vimentin and by hypertrophy of the astrocyte cytoplasmic processes. The astrocytes in certain areas of healthy adult brain tissue, including the cerebellum, retina, and large tracts of myelinated fibers and optic nerve, continue to co-express vimentin and GFAP (Pixley and De Vellis 1984; Calvo et al. 1990; Schmidt-Kastner et al. 1990). Numerous studies have shown in a variety of lesions that reactive astrocytes upregulate GFAP (Bignami and Dahl 1976; Tetzlaff et al. 1988). Although normal astrocytes lose their ability to express vimentin during normal development, reactive astrocytes appear to recover this capacity, especially in close proximity to the site of injury (Pixley and De Vellis 1984; Schiffer et al. 1988). Various experimental models [brain wounds in neonatal (Pixley and De Vellis 1984) and adult (Calvo et al. 1991) rats, for example] have demonstrated the co-expression of GFAP and vimentin by reactive astrocytes. Hozumi et al. (1990) used comparative quantitative immunoblots and immunohistochemistry to show the presence of GFAP-positive cells around the wound 3 h after wounding, unaccompanied howeverby an increase in total GFAP. By 6 h after wounding, GFAP had decreased to 80% of the sham-operated control value, but began to increase again by 24 h. Reactive glia in the vicinity of the wound increased steadily in number and intensity, peaking at about 7 days, then declining significantly by 21 days. A number of experimental models have shown increased immunostaining of GFAP in gliosis, after stab wounding for example (Miyake et al. 1988).
Astrocytes proliferate dramatically from day 0.5 to day 3 after experimental stab wounding (Miyake et al. 1992 see also Janeczko 1989). Maximum numbers of proliferating cells (an increase of GFAP-positive astrocytes) were observed on days 2.5 and 3. The phenomenon of astrocyte proliferation is however relatively limited, being confined mainly to the injury site (Miyake et al. 1992).
3.2.3.2
Piloid Gliosis
Piloid gliosis (also termed pilocytic or isomorphic gliosis) is associated with long-standing degeneration of nerve fibers in, among other sites, the spinal cord. The unusually long fibrils course in pathways parallel to the degenerating nerve fibers.
3.2.3.3
Metabolic (Protoplasmic) Gliosis
Metabolic gliosis (Norenberg 1996) develops mainly in the basal ganglia and cerebral cortex of patients in hepatic or uremic coma or in a precoma lasting several days, especially in victims of hyperammonemia. The astrocytes associated with metabolic gliosis are called “Alzheimer‘s type II astrocytes” (Fig. 3.4g). In metabolic diseases (Adams and Foley 1953) they exhibit a tendency to aggregation (giving rise to socalled Gliarasen) with an increase in number and size of their nuclei, which occur in clusters or pairs as a sign of the proliferation process. The nuclei are vesicular and may show a twofold increase in size. They have a prominent nuclear membrane and an optically empty nucleoplasm whose scant chromatin particles resemble nucleoli. Some of the astrocytes are located in close proximity to neurons (neuronal satellitosis) as a sign of neuronal damage. Intranuclear inclusions may be found upon staining for glycogen [e.g., Periodic Acid Schiff (PAS) stain]. Some but not all of the astrocytes express GFAP. Metabolic gliosis does not exhibit glial fiber formation.
3.2.3.4
Regressive Alterations
Astrocytes do not undergo progressive alterations alone, but also regressive changes such as atrophy, pyknosis, and clasmatodendrosis (Fig. 3.4h). In the early phase following mechanical loading or ischemic injury, the cell body swells and becomes rounded. Later lipid granules and vacuoles appear in the cell body and the surface becomes irregular. The cytoplasmic processes break off (clasmatodendrosis) and disintegrate into granular debris. Astroglia possessing pseudopodia-like appendages alone are called ameboid glial cells.
|
|
3.3
Oligodendrocytes
3.3.1
Morphology, Classification, and Immunoreactivity
Oligodendrocytes are small cells with a round or oval, relatively dense nucleus and a small rim of cytoplasm
with a cell diameter of 6?8 ?m. Oligodendrocytes possess ramified processes that can be demonstrated by silver techniques. They also exhibit a structural polymorphism that reflects differences in function. Three to five types of oligodendrocyte can be distinguished based on localization, which may also be related to differences in function.
1. Perineuronal oligodendrocytes (gray matter oligodendrocytes ? Fig. 3.5a, b). This type of oligodendrocyte is commonly located near the larger pyramidal cells of the cerebral cortex and the nerve cells of the basal ganglia. Perineuronal oligodendrocytes are considered to be analogous to capsule cells of the dorsal root ganglia. They constitute 51% of the total perineuronal glial population (Bunge 1968).
2. Perivascular oligodendrocytes (gray matter oligodendrocytes).Fairly numerous, these cells are seen mainly around cerebral cortical vessels.
3. Interfascicular oligodendrocytes (white matter oligodendrocytes ? Fig. 3.3c, d). This is by far the most common type of cell in the white matter. These have processes that run parallel to nerve fibers and partly or completely encircle nerve fibers; the cell nuclei are disposed in rows. In thecorpus callosum of the rat 69.8% of glial cells in these rows are oligodendrocytes (Mori and Leblond 1970).
4. Szuchet (1995) describes a fourth type of oligodendroglia according to Rio Hortega (1956) which is associated with large axons and located near the entrance of nerve roots into the CNS.
5. An oligodendrocyte precursor cell is described asa fifth type, which comprises 5?8% of the glial cell population in the CNS (Levine et al. 2001). These cells have small cell bodies with multiple branched processes. In gray matter, the processes tend to be oriented radially, in white matter they are more longitudinally arranged and aligned with nerve fibers. Under normal circumstances the processes are in contact with synapses and nodes of Ranvier, a possible indication that these structures play a regulatory role in the axonal impulse conduction.
6. Meanwhile the oligodendrocyte has served as a model for lineage development in part due to the identification of specific additional phenotypic
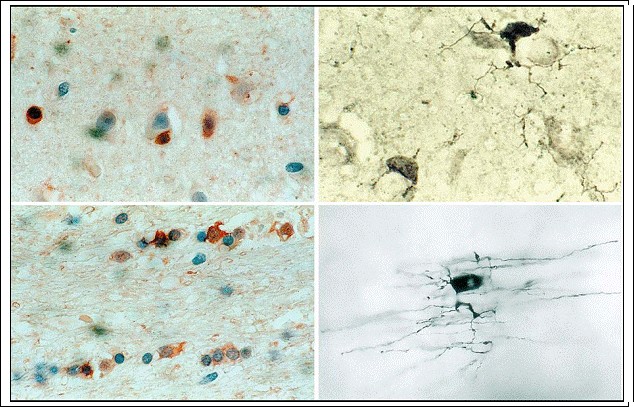
Fig. 3.5a?d. Oligodendrocytes. a, b Perineuronal oligodendrocytes = gray matter oligodendrocytes (a carbonic anhydrase II = CA II; magnification Х500; b silver stain; magnification Х1,000); c, d interfascicular oligodendrocytes = white matter oligodendrocytes
(c CA II; d silver stain; magnification c Х500, d Х1,000)
stages during maturation (Grinspan 2002). The result is the identification of numerous signaling molecules inducing oligodendrocyte development. Oligodendrocytes can be demonstrated by the silver technique and by antibodies to myelin basic protein (MBP), myelin oligodendrocyte glycoprotein, platelet- derived growth factor, galactocerebroside, or to proteolipid protein (Levine et al. 2001; for review, see Friedman et al. 1989). Also specific for oligodendrocytes are the monoclonal antibodies Rip (Friedman et al. 1989), Otex 1 (Mori de Moro et al. 1990), and CC-1 (Bath et al. 1996). In addition, oligodendrocytes have been found to selectively express carbonic anhydrase II (CA II) (Cammer et al. 1977; Ghandour et al. 1980), gluthathione-S-transferase, isoenzyme pi (Tansey and Crammer 1991), and cell-surface sphingolipids, such as galactocerebroside (Raff et al. 1978).
3.3.2
Function
The function of oligodendrocytes is still largely unknown. Different functions have been attributed
to the various types of oligodendrocyte.
1. Peters et al. (1991) propose that perineuronal oligodendrocytes may contribute to neuronal nutrition. It has also been suggested that under certain conditions, such as in remyelination, these satellites are able to produce myelin (Ludwin 1978; Polak et al. 1982). The total amount of myelin oligodendrocytes are able to synthesize is thought to remain relatively constant (Blakemore 1981). It is on the basis of these findings that neurons are considered to be the regulators of myelination.
2. Interfascicular oligodendrocytes are known to be involved in the myelination and remyelinating
processes (Harrison and McDonald 1977). In the early stages of myelin formation the cytoplasmic processes sequentially ensheathe the axons. Following demyelination, the myelin sheath regenerates chiefly by forming near internodes. Before remyelinated fibers may be recognized (within 3 weeks) demyelinated axons are surrounded by processes from one or more cell types, such as astrocytes and debris-laden mononuclear phagocytes. None of these cell types, however, is able to produce a membrane that spirals around the axons or which is compacted in a myelin-like fashion. The regenerated myelin sheaths are formed by oligodendrocytes, which possess microtubules but no filaments (Harrison and McDonald 1977).
3. Little is known about the function of perivascular oligodendrocytes.
4. Oligodendrocyte precursor cells have processes that are in contact with synapses and the nodes of Ranvier, an indication, as mentioned above, that these structures fulfill a regulatory function in transmitting information via bioelectric signals (Levine et al. 2001). Although they divide slowly, these cells constitute about 70% of cells labeled by a pulse injection of bromodeoxyuridine (labelingindex: 0.2?0.3%; cf. Horner et al. 2000).
5. Oligodendrocytes as a cell group also play an active role as an antigen-presenting cell type and
are thus involved in immunological processes. Though oligodendrocytes are negative for MHC class I and II expression in normal human CNS (Sedgwick and Hickey 1997), under pathological conditions oligodendrocytes may show an increase in class I MHC expression (Benveniste 1997). This is because INF-?, normally produced by activated T-cells and absent from the intact CNS,
becomes a potent modulator of MHC antigen expression under pathological conditions, such as T-cell infiltration or disrupted blood?brain barrier.
6. No systematic studies have yet clarified the influence of neurons or astrocytes on oligodendrocytes. Fulcrand and Privat (1977) thought oligodendrocytes need neuronal input, but they did not examine whether there must be physical contact between the two cell types or whether neurons influence oligodendrocytes via secreted factors. Astrocytes and oligodendrocytes are coupled in situ by gap junctions (Massa and Mugnaini 1982). Oligodendrocytes can perform their myelination repertoire without gap junctions, which suggests that these junctions fulfill a function not necessarily directly related to the formation of myelin.
7. Little is known either about the interactions between oligodendrocytes. Oligodendrocytes are aligned in closely apposed rows, the cells being joined by tight junctions (Massa and Mugnaini 1982). This may indicate intense interaction among these cells, the details of which remain unknown.
3.3.3
Pathology
Oligodendrocytes are compromised in many neurological diseases, including demyelinating diseases (e.g., multiple sclerosis), metabolic diseases (e.g., Pelizaeus-Merzbacher‘s disease), infectious diseases (e.g., progressive multifocal leukoencephalopathy), neurodegenerative diseases (e.g., Alzheimer‘s disease), and tumors (e.g., oligodendrogliomas). Under pathological conditions oligodendrocytes assume the various functions described above, the nature of the function partly dependent on the particular cell type. However, this cell type is mainly activated under conditions of myelin degeneration, and their main function is remyelination. Destruction of oligodendrocytes induces unchecked demyelination, with disastrous consequences for brain function. Myelin formation was recently examined by Campagnoni and Skoff (2001) to aid our understanding of oligodendrocyte function. They found that myelin basic protein (MBP) and myelin proteo-lipid protein (PLP/DM20) genes encode classic MBP and PLP isoforms. The products of non-classic MBP isoforms appear to be components of transcriptional complexes in the nucleus of oligodendrocytes. The products of non-classic PLP/DM20 isoforms appear to make up part of the intracellular transport particles within oligodendrocytes. The same authors reported evidence that PLP/DM20 proteins play a role in neuronal death mechanisms, paracrine and autocrine regulation of oligodendrocytes and neurons, oligodendrocyte migration, and intracellular transport. Matsushima and Morell (2001) developed an experimental animal model designed to induce myelin degeneration and remyelination by dietary introductionof the copper chelator bis-cyclohexanone oxaldihydrazone (Cuprizone). After ingestion of the toxin in this model, oligodendrocytes suffer a specific primary insult and undergo apoptosis. Soon thereafter, recruitment of microglia begins and myelin phagocytosis ensues. Next, oligodendrocyte progenitor cells proliferate and invade the demyelinated area. Once the Cuprizone challenge is ended, remyelination commences and is almost completed in a matter of weeks. It can be inferred from their findings that different cell types communicate by soluble factors. Remyelination is to a certain extent accomplished either by surviving oligodendrocytes, or by cells newly differentiated from the adult progenitor pool.
The oligodendrocyte precursor cells in injured CNS constitute a reactive glial population that goes through hypertrophy and mitosis under stimulation from an array of cytokines and growth factors. If there is demyelination, these cells divide and differentiate into new oligodendrocytes that replace the ones that were lost. Activation and proliferation of these cells also occur in response to other types of CNS damage, including excitotoxicity, mechanical injury, and viral infection.Ischemic oligodendroglial injury was recently described in a neonatal rodent stroke model (Liu et al. 2002). The authors showed that myelin proteins are restored in the brain after moderate, but not after severe, cerebral hypoxia-ischemia. Dewar et al. (2003) gave an excellent review on this topic and demonstrated that oligodendrocytes may be targets of injury in acute ischemia. Alterations of their distinct cytologic features and specific immunocytochemical reaction gave evidence of oligodendrocyte damage in animal models. For example, oligodendrocytes became immunoreactive for the cytoskeletal protein tau (Dewar and Dawson 1995; Irving et al. 1996, 2001; Uchihara et al. 2000). Oligodendrocytes have also been shown to inhibit the regenerative response to axonal injury (Schwab 1993 ? see also p. 66).
3.4
Microglia and/or Mononuclear Phagocytes of the CNS
Microglia make 5?12% of the CNS glia (Lawson et al. 1990). If microglia constitute 10% of the total glial cell pool, and if glial cells are at least 10 times as numerous as neurons in the CNS, then microglia are as numerous as neurons (Streit 1995). The proliferative activity, i.e., the microglial turnover rate, is very low (0.05% ? Lawson et al. 1992), which means this cell type has a long life span within the brain tissue. Rio Hortega (1932) ascribed to microglia a mesenchymal origin. Especially during embryonic development, hematopoietic monocytes invade the CNS as well as the CSF and the perivascular spaces, and mature within the brain parenchyma into typical process-bearing resident microglia (Ling and Wong 1993). Only a few authors still question this process (Fedoroff 1995). Research on bone marrow transplantation (BMT) has provided information on the turnover rate of mononuclear CSF cells. When bone marrow cells from male donors are transplanted into female hosts, in situ hybridization with Y-chromosome-specific probes showed that by the time complete donor type hematopoiesis had become established (19?97 days after BMT), all cells within the CSF were donor derived (Hibi et al. 1997). In another study, perivascular cells within the brain contained the donor marker, while parenchymal microglia did not (Unger et al. 1993). These recent findings as former observations (for review see Oehmichen 1978) indicate that mesenchymal cells derived from the bone marrow patrol the CNS continuously and that the perivascular and meningeal macrophage population is slowly replaced by hematogenous macrophages. The population of parenchymal microglia, in contrast, seems to be quite stable in the mature normal brain and may not be replaced by new bone marrow-derived macrophages (Bauer et al. 2001). Additionally we have to point to another cell population within the CNS with similar functional properties as microglia: dendritic cells. Dendritic cells are a subclass of antigen-presenting cells critical in the initiation and regulation of adaptive immunity against pathogens and tumors as well as in the triggering of autoimmunity (for review see Pashenkov et al. 2003). Dendritic cells are present in normal meninges, choroid plexus, and CSF, but absent from the normal brain parenchyma. Inflammation is accompanied by recruitment and/or development of dendritic cells in the affected brain tissue.
3.4.1
Morphology, Classification, and Immunoreactivity Microglia can be differentiated by localization or functional stage. The term “mononuclear phagocyte” (Oehmichen 1978) is well chosen and designates all monocyte-derived cells within the CNS. The following cell types are distinguished (Oehmichen 1978; Hickey et al. 1992; Gehrmann and Kreutzberg 1995; Perry and Gordon 1997):
1. Leptomeningeal and choroidal macrophages (Fig. 3.6a, b). These cells are phenotypically macrophages, express macrophage antigens, and are localized within the subarachnoid space and ventricular system. They have a relatively high replacement rate (60% ? Hickey et al. 1992).
2. Perivascular macrophages (Fig. 3.6c). These are important immunoregulatory cells to be distinguished from so-called pericytes. They are enclosed by a basal lamina, express macrophage markers, and may act as sensors of CNS perturbations. They are activated by CNS inflammation and are primary targets of human immunodeficiency virus (HIV) infection in the CNS (Williams et al. 2001). Their turnover rate is about 30% (Hickey et al. 1992). Leptomeningeal, choroidal, and perivascular macrophages can all be induced to phagocytosis. Unlike other glial cells, even compared to resting microglia, they are efficient and active antigenpresenting cells (Hickey and Kimura 1988; Ford et al. 1995).
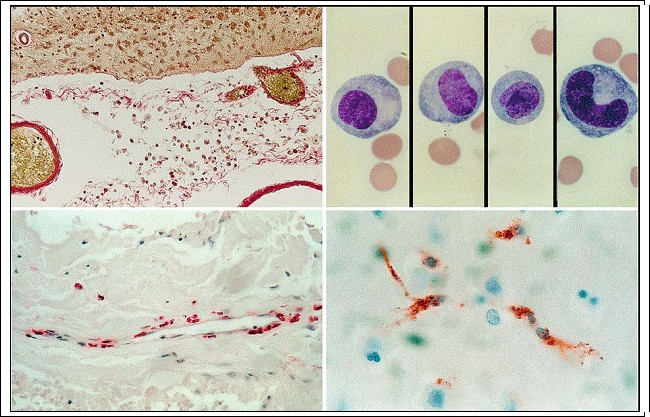
Fig. 3.6a?d. Mononuclear phagocytes. a Subarachnoid macrophages as seen on microscopic sections (v. Gieson stain); b subarachnoid macrophages as seen in CSF specimens (see Oehmichen 1976; Giemsa stain); c perivascular macrophages within Virchow-Robin space (CD68 reactivity); d perivascular microglia in the next vicinity of capillaries (CD68 reactivity; magnification a, c Ч300, b, d x1,000)
3. Perivascular microglia (Fig. 3.6d). These cells form a subtype of resting parenchymal microglia. Perivascular microglia come in direct contact with the adjacent basal lamina and thus, like pe rivascular astrocytic endfeet, form part of the perivascular glia limitans (Lassmann et al. 1991). 4. Ramified resting microglia in the normal CNS (Fig. 3.7). This cell type has a small heterochromatic nucleus and fine, ramified processes which exhibit a stellate morphology in the gray matter and a bipolar, longitudinal morphology in the white matter. Under pathological conditions they may transform into “activated microglia” (see below). Leptomeningeal, choroidal, and perivascular macrophages exhibit the same immunoreactive pattern as macrophages and activated microglia and may be termed “mononuclear phagocytes.” Ramified resting microglia are demonstrated by the silver carbonate method (Rio Hortega 1919), by immunohistochemical procedures (for review see Gehrmann and Kreutzberg 1991; Streit 1995), and by histochemical techniques (Oehmichen 1980). As late as the 1990s it was thought that the blood?brain barrier prevented macrophages from invading the CNS. It was observed that a disrupted blood?brain barrier did not invariably result in invasion of the CNS parenchyma by monocytes. In addition it was noted that circulatory monocytes cross the intact blood?brain barrier in response to the degeneration of other cell types or to inflammatory substances within the parenchyma. Like activated Tlymphocytes, monocytes are also known to cross the intact blood?brain barrier under non-pathological conditions (Hickey 1999). For a long time, the demonstration of resting and activated microglia only succeeded due to silver techniques. Selective immunohistochemical staining is only possible because of the expression of specific surface proteins in microglia. Visualization in vivo is possible (Banati 2002) using 11C-labeled ligands for the peripheral benzodiazepine binding site (PBBS), which binds to activated but not resting microglia with relatively cellular selectivity.
3.4.2
Pathology
Under pathological conditions a cell type appears which stains like resting microglia, the so-called activated microglia. Activated resting microglia in animal experiments (mice) express OX-42 and CR3- complement receptors (Graeber et al. 1988a) plus F4/80 and Mac-1 antigen (Perry et al. 1985).
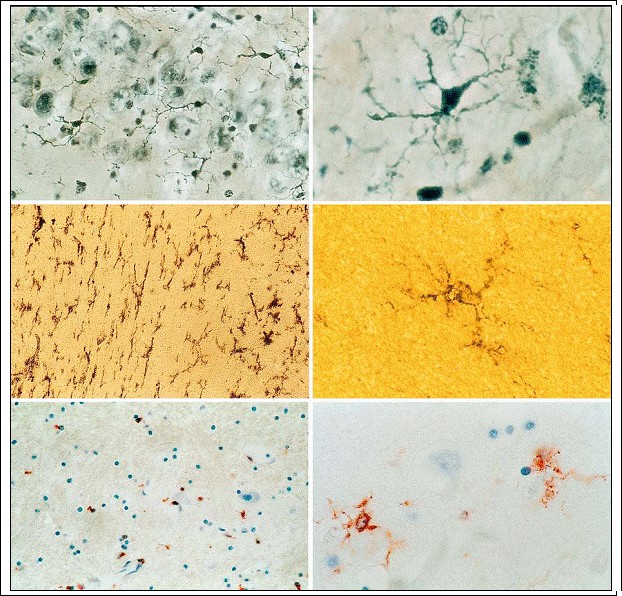
Fig. 3.7a?f. Ramified resting microglia in normal CNS. a, b Silver stain; c, d UDPase; e, f CD68 reactivity (magnification a, c, e x500; b, d, f x1,000)
In pathological human brain tissue, activated microglia can be detected in routine paraffin material using the monoclonal antibody LN-3 (Sasaki et al. 1991). Activated microglia also express MHC class I and II antigens (Streit et al. 1989) in addition to almost all other macrophage-specific antigens.
Activated microglia possess an ability to proliferate locally, to emigrate to the site of injury, to change morphologically, immunophenotypically, as well as functionally (Gehrmann and Kreutzberg 1995). Two types of activated microglia are recognized:
- Activated, non-phagocytic microglia (Fig. 3.8a). These cells are hypertrophic and have processes which are more numerous than those of resting microglia. They express vimentin, several macrophage markers, including CR3 complement receptor, MHC class I and II, and CD4 antigen (Streit and Kreutzberg 1987). They are characterized by intense upregulation of CD68 and CD14 (Beschorner et al. 2002).
- Activated phagocytic microglia (Fig. 3.8b-d). Morphologically these cells are characterized by an ameboid cell structure lacking processes as seen after traumatic (Fig. 9.8) or ischemic events (Fig. 14.12?14.15); functionally they resemble non-phagocytic microglia, but are additionally capable of phagocytosis of neurons (Fig. 3.8e), of fat (Fig. 3.8f), of myelin (Fig. 3.3d), etc. and are
capable of releasing immunomodulatory compounds. Microglia may respond to sterile CNS in-
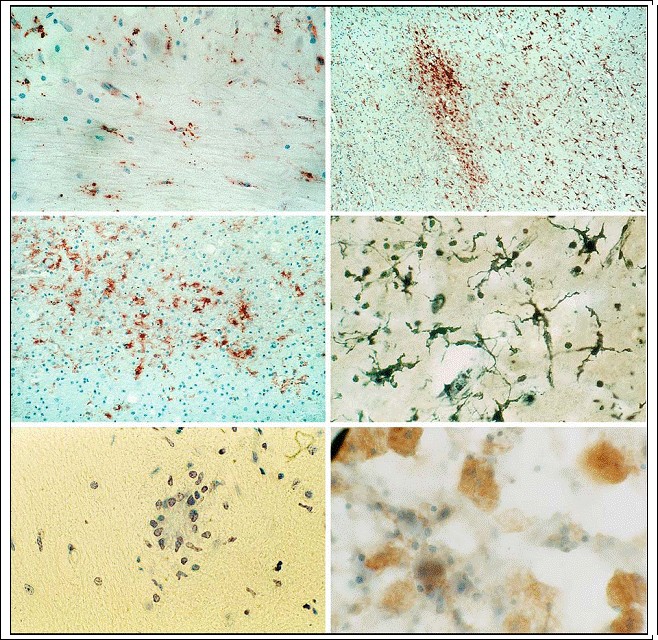
Fig. 3.8a-f. Activated microglia. A moderate (a) and a distinct cluster-like increase (b, c) of activated microglia demonstrated by CD68-immunohistochemistry as well as by silver stain (d) (magnification a Ч500; b x100; c x500; d x1,000); e perineuronal aggregation of microglia (satellitosis or microglial nodule) as an indication of neuronal phagocytosis (Nissl stain; magnification x1,000); f lipid-containing phagocytes (Fettkцrnchen-Zellen) as an indication of the scavenger function of microglia (oil-red 0; magnification x1,000)
jury as a consequence of their scavenger function, such injury also stimulating a release of growth factors that participate in the scarring process of gliosis. Graeber et al. (1997) suggested that microglia are a “sensor” of the pathological status of the human CNS. Under certain physiological and especially pathological conditions of the CNS microglia react very quickly. Microglia are slightly more numerous in elderly brains and they show more phagocytotic activity (Peters et al. 1991). Under conditions of dehydration, microglial cells proliferate in the posterior pituitary and the supraoptic nucleus (Lawson et al. 1993). It has recently been shown that monocytes aggravate neuronal degeneration, for example in cases of HIV-infected macrophages which produce a neurotoxic substance (Giulian et al. 1990). In other conditions involving rapid recruitment of macrophages, they secrete neurotoxic compounds (Piani et al. 1991) and can damage myelin (Coffey et al. 1990 ? for review see Giulian 1995). Two observations have potential practical (forensic) applications: Lassmann (1997) used the detection of myelin degradation products within brain macrophages to estimate the age of trauma-induced demyelination (1?14 days), and thus to stage lesions of CNS white matter (cf. Oehmichen et al. 1986). Meyermann et al. (1997) applied various immunoreactive macrophage markers to show that although MBI may induce upregulation of various antigens, it does not induce macrophage proliferation at the margins of mechanically induced hemorrhages ? in contrast to the processes resulting from an ischemic lesion. A sophisticated questioning of the functional potential of microglia was given by Streit (2002). He examined the possible consequences of microglial dysfunction as a result of aging, genetics, or epigenetics. He supposed that microglial senescence was a potential factor in the pathogenesis of Alzheimer‘s disease. His hypothesis is based on observations of microglial abnormalities, especially microglial deramification, spheroid formation, gnarling and fragmentation of processes in Alzheimer‘s disease (Streit et al. 2004), A further question must be answered: the fate of activated microglia. Obviously, a part of activated microglia will leave the CNS by migrating along lymphatic pathways (Oehmichen et al. 1979b). Another portion may disappear by focal cell death induced by interleukin-13 (IL-13) in association with IL-4 in a time-dependent manner (Yang et al. 2002).
3.4.3
Function
A cell type‘s immunophenotypical properties are an indication of its phagocytic and immunological functions (Oehmichen 1983; Gehrmann and Kreutzberg 1995).
1. Resting microglia as well as the group of mononuclear phagocytes upregulate Fc and complement receptors (Oehmichen 1978), which could also be demonstrated in resting microglia using a monoclonal F4/80 antibody (Perry et al. 1985).
2. Resting microglia normally lack MHC class I and only a few microglial cells express MHC class II (Perry and Gordon 1997). The immunological situation created by inflammatory processes (neuritis, brain abscess, glioma, viral infection, neurotoxin lesion) leads to the activation of resting microglia and infiltration of the CNS by blood monocytes, which express MHC classes I and II.
3. Recent investigations give evidence of the expression of “scavenger” receptors (class A, Bi and CD36) by microglia, astrocytes, cerebral microvascular endothelial cells, etc. (Husemann et al. 2002). These receptors are cell surface proteins that mediate cell adhesion to, and endocytosis of, various native and pathologically modified substances, and participate in intracellular signaling, lipid metabolism, and host defense against bacterial pathogens. Neonatal microglia express scavenger receptors, while these receptors are not expressed in normal mouse or human adult brain microglia.
4. Activated microglia synthesize tumor necrosis factor (TNF) in the very early phase (minutes to a few hours) after induction of ischemia (Lambertsen et al. 2002). TNF is a potential neurotoxic cytokine.
5. Activated microglia upregulate vimentin (Graeber et al. 1988b) and both MHC classes I and II, ED-1, etc. (see above).
6. Activated microglia perform all of the immunological functions attributed to macrophages in sterile and non-sterile inflammation: they release immunomodulatory compounds and interact with other immunocompetent cells. Moreover, the functional potential of microglia is not limited to their surface protein expression but it is additionally characterized by their interaction with cytokines (for review see Hanisch 2002). Cytokines constitute a significant portion of the immuno- and neuromodulatory messengers that can be released by activated microglia. By virtue of potent effects of resident and invading cells, microglial cytokines and chemokines regulate innate defense mechanisms, help the initiation and influence the type of immune response, participate in the recruitment of leukocytes to the CNS, and support attempts at tissue repair and recovery. Moreover, microglia can also receive cytokine and chemokine signals as part of their communication with astrocytes, neurons, endothelium, and leukocyte infiltrates. The inflammatory response, therefore, is marked by the appearance of phagocytes, which arise from activated resting microglia as well as from invading monocytes, which bear scavenger receptors within 5?8 h after wounding (Giulian et al. 1993). In the rat brain their numbers were found to peak 2 days after the traumatic event. Meanwhile there is additional evidence of the reciprocal interactions between microglia and neurons and complex signaling systems (Polazzi and Contestabile 2002). The signal relates to the suppression of immunological properties of microglia by neurons in the healthy brain and between damaged neurons and microglia. The authors propose that microglial activation, consequent to neuronal injury, is primarily aimed at neuroprotection (Polazzi and Contestabile 2003). The loss of specific communication between damaged neurons and microglia is viewed as being responsible for the transformation of microglia to a hyperactive state, allowing them to escape neuronal control and giving rise to persistent inflammation, resulting in exacerbation of neuropathology.
3.4.3.1
Resting Microglia
It is still not known exactly what function resting microglia perform within normal brain parenchyma. They may participate in tissue homeostasis and in the early defense against injury and infection (Gordon et al. 1988; Perry and Gordon 1997). Because resting microglia and mononuclear phagocytes cross react, using different antibodies, resting microglia seem to take up the same functions in the CNS as resting macrophages in the tissue of other parenchymal organs, i.e., both a scavenger function and an immunological function. The most important function appears to be their transformation under pathological conditions into intrinsic phagocytes. Their ability to be activated is expressed above all in their capacity to proliferate, to phagocytose, and to release immunomodulatory compounds. Microglia form a network of antigen-presenting cells whose main function is immune surveillance (Oehmichen 1983; Perry and Gordon 1997). Under pathological conditions resting microglia change into mobile, phagocytic cells that are capable of neuronophagia (Banati et al. 1993) and which release proteases, proinflammatory cytokines (IL-1, TNF-?, IL-6, etc.), anti-inflammatory cytokines (IL-10, TGF-?), cytotoxic molecules (nitric and oxygen radicals), prostanoids (PGD2, PGE2, thromboxane B2), and chemokines (IL-8, etc.) (Aloisi2001).
3.4.3.2
Perivascular Microglia
Perivascular microglia apparently participate in functions of the blood?brain barrier system. Together with leptomeningeal phagocytes, they fulfill a scavenger function within the CSF, especially under pathological conditions such as hemorrhage, stroke or infection (for review of literature up to 1982 see Oehmichen 1982b; 1982?1995: see Gehrmann and Kreutzberg 1995). They are involved in the immunoreactive response as they are antigen-presenting cells. Some data appear to indicate that perivascular microglia return to the spleen and lymph nodes bearing the material they have gathered in the CNS (Oehmichen et al. 1982a, b; Broadwell et al. 1994). Direct labeling of a particulate antigen in the CNS, however, could not show that these cells triggered an immune response to the antigen (Matyszak and Perry 1998, cf. Graeber et al. 1992).
3.4.3.3
Activated Microglia (Macrophages)
Activated non-phagocytic microglia is involved in immunological processes as a regulatory cell in the defense against pathogens and tumor. Activated phagocytic microglia phagocytose necrotic cells ? especially necrotic neurons (Fig. 3.8e), cell particles, and other debris resulting from, e.g., edema, myelin degradation (Figs. 3.3d, 9.15), necrosis, i.e., lipids (Figs. 3.8f, 9.16), or red blood cells in hemorrhages (Figs. 9.17, 9.18). Activated microglia may ingest extravascular erythrocytes, which can be demonstrated
as erythrocyte- or siderin-bearing macrophages (Figs. 9.17, 9.18). They may also contain ingested nuclear material, myelin, and lipids, which are changed by intracellular digestion (Oehmichen et al. 1986; Lassmann 1997). In special cases the ingested substances may enable retrospective diagnosis and timing.
3.5
Additional Cell Types and Tissue Components
The cell types and different tissues described in the following protect the CNS parenchyma from extracerebral influences, such as blood constituents and microorganisms, and thus maintain intracerebral homeostasis (for a survey of these cell types see Bargmann et al. 1982).
3.5.1
Leptomeningeal and Perivascular Cells
The leptomeninges are composed of the two more delicate components of the meninges, the pia mater and the arachnoid mater, considered together. The pia mater contains the blood vessels that penetrate the brain, while the arachnoid mater is composed of the arachnoid membrane and trabeculae, the latter stretching from the membrane to the pia. Since this situation arises from a progressive enlargement embryologically of the extracellular space of the originally solid leptomeninges, the arachnoid trabeculae are surrounded by the subarachnoid space containing CSF. The term pia mater is applied to the entire brain and spinal cord surface. Moreover, funnel-shaped pia mater accompanies vessels that extend into the brain. The undersurface of the pia mater possesses a well-defined glial basement membrane. The portion of the glial membrane that courses along the penetrating vessels is called the membrana gliae perivascularis. The arachnoid membrane constitutes the outer limit of the arachnoid. The membrane spans the sulci of the cerebrum and cerebellum and lies near the brain stem. The arachnoid trabeculae composed of collagen fibrils, fibrocytes, and fibroblasts span the subarachnoid space and attach the arachnoid membrane to the pia mater. The vessels entering the cortex are thin-walled, comprised merely of a basement membrane, endothelium, and a single layer of smooth muscle cells. The Virchow-Robin space (perivascular space) lies between the vessel basement membrane and the glial basement membrane. Both the subarachnoid space (Fig. 3.6a, b) and the Virchow-Robin space (Fig. 3.6c) are filled with CSF, which contains single macrophages and isolated lymphocytes (Oehmichen 1976). Two-thirds of the CSF is produced by the choroid plexus. To reach the subarachnoid space, CSF courses from the lateral to the third ventricles through the interventricular foramina (of Monro), the third ventricle, the cerebral aqueduct of the midbrain, the fourth ventricle, the two lateral foramina of Luschka and the midline foramen of Magendie. It exits the intracranial spaces via arachnoid villi or via lymph channels at the level of the nerve roots (for review: Oehmichen et al. 1982a, b).
3.5.2
Parenchymal Vessels
Parenchymal vessel walls of the brain are very similar in construction to those of other organs (for details, see Chap. 28, pp. 542 ff). Normal arteries possess an intimal layer composed of endothelial cells with longitudinally oriented nuclei and perikarya. A basement membrane covers the endothelial cells. The internal elastic lamina separates the intimal layer from the lamina media, which consists of smooth muscle cells. The lamina adventitia is formed of loose connective tissue containing collagenous fibers, pericytes, fibroblasts, and perivascular macrophages, which are typical of the Virchow-Robin space. The normal veins of the brain are characterized by a wide lumina and relatively thin vessel walls. They have no internal elastic lamina. Brain capillaries differ from those of other organs by their complete or almost complete impermeability (blood?brain barrier) and lack of fenestrations. The neighboring endothelial cells are bound together by tight junctions or zonulae occludentes, which prevent the passage of tracer substances. This barrier function is absent in vessels of the choroid plexus, pineal body, pituitary gland, area postrema, tuber cinereum, and median eminence. Under pathological conditions, shrinkage of endothelium can occur and thus leads to the formation of fenestrations. The tight junctions can also fail, or pinocytotic vesicles may arise within the endothelial cells, all of which are associated with a breakdown in the blood?brain barrier (cf. Chap. 4, pp. 42f). Endothelial cells may proliferate, as seen in glioblastoma, or as a consequence of ischemic and/or toxic damage to the CNS.
3.5.3
Choroid Plexus and Ependyma
The cells of the surface of the choroid plexus (choroidal cells) as well as of the ventricular system (ependymal cells) originate from neuroectodermal ependymoblasts of the neural tube. The choroid plexus possesses fronds produced by elaborate infolding of the plexus surface. The fronds exhibit ramified villous processes, each supported by connective tissue. The choroid plexus has clearly defined projections into the third, fourth, and lateral ventricles of the brain. Its surface is covered by a thin epithelium composed of low columnar, cuboidal, or squamous cells mounted on a basement membrane. Choroidal cells secrete two-thirds of the CSF (roughly 500 ml/ day in an adult), the remaining one-third (roughly 250 ml/day in an adult) arising from the interstitial fluid of the brain. The choroidal CSF can be thought of as fresh fluid diluting and rinsing the more stagnant and metabolite-laden extrachoroidal, tissuederived CSF. The ventricular surfaces are lined by cuboidal to columnar cells with cilia and microvilli, the ependymal cells. Adjacent cells are bound to each other by desmosomes. The hypothalamic wall of the third ventricle contains a specialized ependymal cell type called the tanycyte. The functions of ependymal cells are secretion, absorption, transport, receptor tasks, and provision of a barrier between the CSF and brain (Del Bigio 1995).
3.5.4
Pathology of the CSF and Cells Within the CNS
Disturbance of CSF turnover can lead to a pathological reaction in the form of hydrocephalus . The turnover can be disturbed by disruption of CSF reabsorption, particularly by diseases of the arachnoid villi. Subarachnoid hemorrhage due to an efflux of red blood cells can cause blockage of the arachnoid villi, and thus a backup of CSF, while the ventricle to CSF pathways for CSF flow remains open (communicating hydrocephalus). Disturbance of the pathways communicating between the ventricular system and subarachnoid space may cause internalhydrocephalus (non-communicating hydrocephalus). The pathways can also be congenitally blocked or impeded by an atresia (congenital non-communicating hydrocephalus) or the blockage can result from infection (postmeningitic hydrocephalus) or tumor, giving rise in each instance to an obstructive, i.e., high pressure, hydrocephalus. Disease processes, e.g., following mechanical violence or stroke, can lead to a reduction in the brain parenchyma, producing a so-called hydrocephalus ex vacuo. All of the above mentioned cell types discussed here line the surfaces of the brain and protect it against extracerebral influences (Adams et al. 1982). The vessels of the brain are especially well suited by their barrier function to guarantee homoeostasis within the brain parenchyma. The CNS was once thought to be an immunologically privileged organ, exempted from immune reactivity and immune surveillance. This is attested to by the absence of MHC molecules basic to antigen presentation from resident cells of the nervous system, but not from perivascular cells and CSF macrophages. Under normal circumstances the aforementioned resting microglia are present in the CNS and subject to activation. Normal CSF contains a few lymphocytes (Oehmichen 1976), and activated (but not resting) T-lymphocytes can cross the blood?brain barrier to carry out immune surveillance of the normal brain parenchyma (Wekerle et al. 1986). A constant surveillance of the intact healthy CNS is performed by activated T-cells. Recent investigations show that B-lymphocytes enter all parts of the normal brain in very low numbers and that the B-lymphocytes within the brain parenchyma display an activated (CD23 positive) phenotype (Anthony et al. 2003). Under pathological conditions, the situation changes considerably, the alterations involving both the endothelial cells and leukocytes (cf. Chap. 29, pp. 582 ff). The CNS is subjected to massive infiltration by immune cells (Flьgel and Bradl 2001; Hickey 2001) dependent on antigens (Hickey et al. 1997) and release of various cytokines (Benveniste 1997). When attachment and transendothelial migration of blood-derived immunocompetent cells occur, cell surface glycoproteins called adhesion molecules, expressed on both lymphocytes and endothelial cells, exert extensive control over the leukocytes. When emigration from the vascular compartment into the extravascular compartment is to occur, leukocytes first establish loose contact with endothelial cells via C-type lectins, or selectin molecules, especially the P-selectin (CD62), which appears within minutes after a mechanical or ischemic insult on the surface of endothelial cells. Attachment of the leukocyte to the endothelial cell is mediated by adhesion molecules such as the intercellular adhesion molecules (ICAMs) and the vascular cell adhesion molecule-1 (VCAM-1). Integrins such as the lymphocyte function- associated antigen-1 (LFA-1) may contribute to a cell‘s ability to migrate out of a vessel. The endothelium of the CNS normally expresses no or only low levels of adhesion molecules. If there is inflammation of the CNS, adhesion molecules are widely expressed. TNF-?, a cytokine released by macrophages, plays an especially crucial role in the induction of adhesion molecules. Once leukocytes have entered, T-cells (and possibly B-cells) recognize their antigen, thus initiating the next step in the induction of inflammation. Antigen-presenting cells other than astrocytes, oligodendrocytes, and endothelial cells include perivascular cells, microglia, and leptomeningeal macrophages. In macrophages, proinflammatory cytokines upregulate the antigenpresenting function. The chief cytokine in this regard is IFN-?, which is released by activated T-cells (Sedgwick and Hickey 1997). The role of complement in the emigration of leukocytes in inflammation and injury is still not entirely known (Morgan 1999). Administration of the complement inhibitor sCR1 just before brain injury has been shown to greatly inhibit infiltration of neutrophils into the injured area, an indication that local activation of complement contributes to inflammation(Kaczorowski et al. 1995). In aneurysm rupture causing cerebral hemorrhage, complement activation is combined with potentially life-threatening cerebral vasospasm (Ostergard et al. 1987). Complement activation and reperfusion injury may follow transient cerebral ischemia in stroke and transient ischemic attacks, with fatal consequences (Czurkoand Nishino 1994).
Bibliography
Adams RD, Lee JC (1982) Neurons and neuronal reactions in disease states. In: Haymaker W, Adams RD (eds) Histology and histopathology of the nervous system. Charles C Thomas,Springfield, Ill., pp 174-275
Graeber MB, Blakemore WF, Kreutzberg GW (2002) Cellular pathology of the central nervous system. In: Graham DI, Lantos PL (eds) Greenfield‘s neuropathology, vol 1. Arnold, London, pp 123?191
Kettenmann H, Ransom BR (eds) (1995) Neuroglia. Oxford University Press, New York
Lindenberg R (1982) Tissue reactions in the gray matter of the central nervous system. In: Haymaker W, Adams RD (eds) Histology and histopathology of the nervous system, vol 1. Charles C Thomas, Springfield, Ill., pp 973-1275
|